Abstract
Objectives: Our aim is to assess the subfield-specific histopathological correlates of hippocampal volume and intensity changes (T1, T2) as well as diff!usion MRI markers in TLE, and investigate the efficacy of quantitative MRI measures in predicting histopathology in vivo.
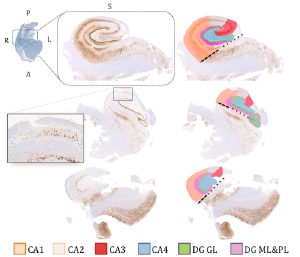
Experimental Design: We correlated in vivo volumetry, T2 signal, quantitative T1 mapping, as well as diffusion MRI parameters with histological features of hippocampal sclerosis in a subfield-specific manner. We made use of on an advanced co-registration pipeline that provided a seamless integration of preoperative 3 T MRI with postoperative histopathological data, on which metrics of cell loss and gliosis were quantitatively assessed in CA1, CA2/3, and CA4/DG.
Principal Observations: MRI volumes across all subfields were positively correlated with neuronal density and size. Higher T2 intensity related to increased GFAP fraction in CA1, while quantitative T1 and diffusion MRI parameters showed negative correlations with neuronal density in CA4 and DG. Multiple linear regression analysis revealed that in vivo multiparametric MRI can predict neuronal loss in all the analyzed subfields with up to 90% accuracy.
Conclusion: Our results, based on an accurate co-registration pipeline and a subfield-specific analysis of MRI and histology, demonstrate the potential of MRI volumetry, diffusion, and quantitative T1 as accurate in vivo biomarkers of hippocampal pathology.